Cancer, heart disease vaccine could be ready by 2030
Fox News medical contributor Dr. Janette Nesheiwat says the technology that the vaccine uses has been studied since the 1980s and that it teaches the body to recognize cancerous cells in order to attack and destroy them.
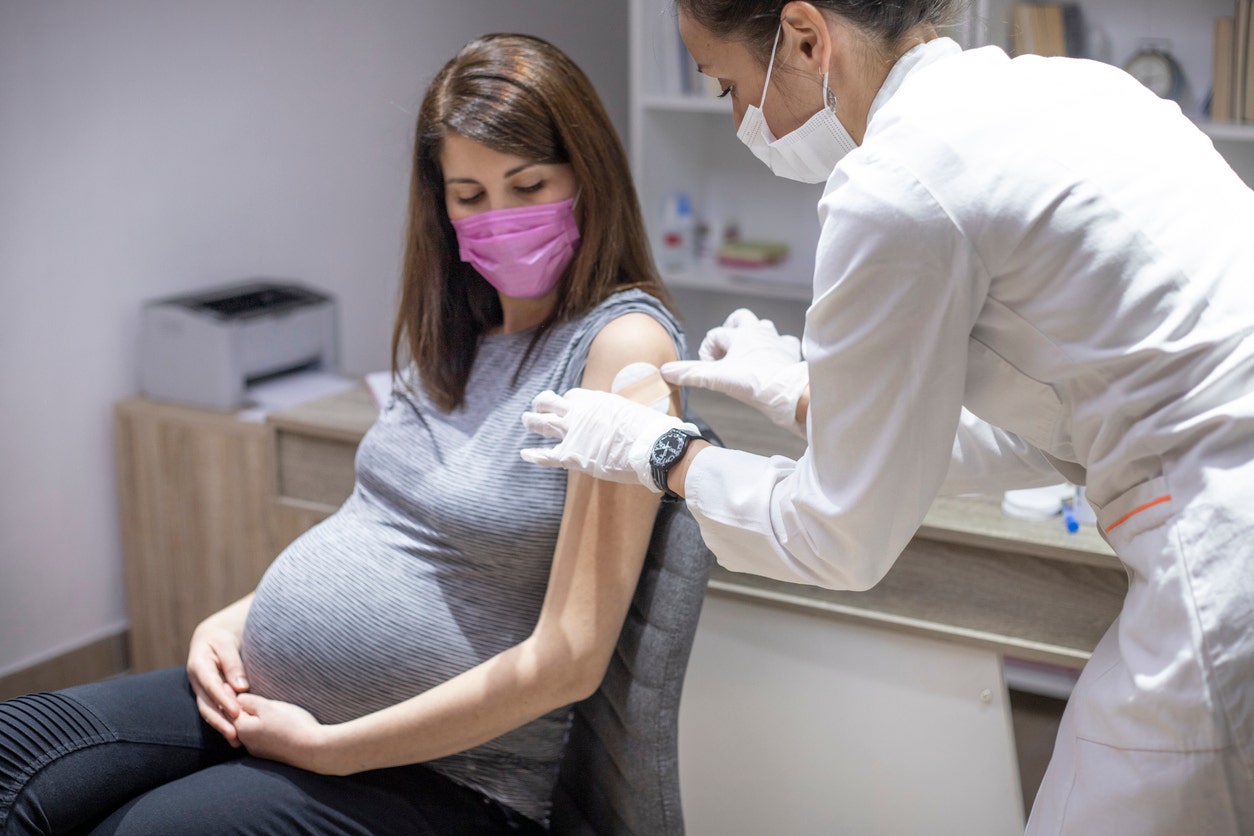
The U.S. Food and Drug Administration (FDA) voted on Thursday to recommend approval of Pfizer’s RSV vaccine for pregnant women. The vaccine is now one step closer to becoming the first available maternal immunization to protect infants from the respiratory syncytial virus.
After a day-long public meeting held via online conference, the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted unanimously (14-0) in support of the vaccine’s effectiveness in preventing adverse effects of RSV in infants up to six months of age when given to women during the second or third trimesters of pregnancy.
The committee also voted 10-4 on the safety of the vaccine.
NEW REPORT SUGGESTS ‘PANIC BUYING’ OF MEDICATIONS BY PATIENTS AND PROVIDERS CAUSED DRUG SHORTAGES
“We are encouraged by the outcome of today’s VRBPAC meeting, as it is a critical step forward in the scientific community’s long-sought-after goal to help prevent RSV disease in infants during their most vulnerable first six months of life,” said Annaliesa Anderson, PhD, Pfizer’s senior vice president and chief scientific officer of vaccine research and development, in a media statement.
The U.S. Food and Drug Administration (FDA) has recommended the approval of Pfizer’s RSV vaccine for pregnant women. (iStock)
“If approved, our RSV vaccine candidate has the potential to be the first maternal immunization vaccine to help protect infants at first breath through their first six months of life from this potentially serious infection,” she added.
“Giving it to pregnant women gives passive protection to the fetus, which then carries over to early infancy.”
Before making its decision, the committee reviewed the results of Pfizer’s Phase 3 clinical trial, which were published in The New England Journal of Medicine.
Although the recommendations are a step toward the vaccine’s availability, an official approval from the FDA is still pending.
The agency is expected to make its final decision by August 2023.
Vaccine seems promising, but more research is needed, says doctor
“I think this is a very promising vaccine and is likely very effective and safe, especially with tens of thousands of very young children being hospitalized with RSV every year,” Dr. Marc Siegel, a professor of medicine at NYU Langone Medical Center and a Fox News medical contributor, told Fox News Digital.

Some 58,000 to 80,000 children 5 years of age and younger are hospitalized with an RSV infection, according to data from the CDC. (iStock)
“Giving it to pregnant women gives passive protection to the fetus, which then carries over to early infancy,” he added.
Last week, Siegel interviewed Dr. Paul Offit of Philadelphia, Pennsylvania, who is on the FDA advisory committee.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“He was very positive about the vaccine,” Siegel said. “When he actually saw the full data, however, he ended up voting no [on the safety issue], apparently because of the possible association with pre-term birth.”
Added Siegel, “For me, this means further research needs to be done. I trust Dr Offit’s views on this.”
RSV’s danger to babies
In some cases, the virus poses a danger to infants and toddlers.
Some 58,000 to 80,000 children 5 years of age and younger are hospitalized with an RSV infection, according to data from the Centers for Disease Control and Prevention (CDC).

Infants younger than 6 months old may show symptoms including irritability, lethargy, decreased appetite and pauses in breathing. (iStock)
In most kids, RSV causes only mild cold-like symptoms, but in some cases it can lead to severe respiratory conditions like pneumonia or bronchiolitis, which may require hospitalization and supportive care.
CLICK HERE TO GET THE FOX NEWS APP
The children at the highest risk include premature infants, babies six months and younger, kids younger than two years old who have chronic lung disease or heart disease, children with weakened immunity and those who have neuromuscular disorders, per the CDC.
FIRST-EVER RSV VACCINE APPROVED BY FDA FOR ADULTS 60 AND OVER
Infants younger than six months old may show symptoms including irritability, lethargy, decreased appetite and pauses in breathing.
Earlier this month, it was reported that the FDA approved the first RSV vaccine, called Arexy, for use by people 60 years and older to prevent the lower respiratory tract disease.